Structure & Design

Optimized design
Manufactured from cobalt-chromium (CoCr) with a thin thickness of 75μm, the stent provides an excellent crossing profile and robust radial force. Its hydrophilic delivery system enhances flexibility and supports high balloon burst pressure. The abluminal coating combines PLA and PLGA polymers with an optimized dosage of Sirolimus, creating a moderate drug elution profile that promotes rapid and uniform endothelialization.
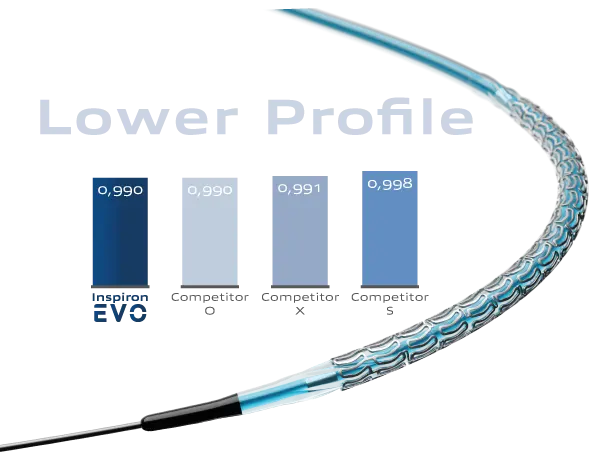
The EVO drug-eluting stent features a significantly reduced profile compared to its previous generation, enhancing its ability to cross lesions with exceptional ease.
Abluminal coating
Layer thickness of 5 μm
Rounded structure designed to minimize the risk of arterial damage
I features “S” connectors that enhance flexibility and navigability
Optimized design with slim structures
Optimized design with slim structures
Cobalt Chrome Alloy of 75 μm thickness structure
Exceptional radial force)

Hydrophilic coating
High nominal burst pressure

Optimized design with slim structures
Cobalt Chrome Alloy of 75 μm thickness structure.
Exceptional radial force
Clinical Studies
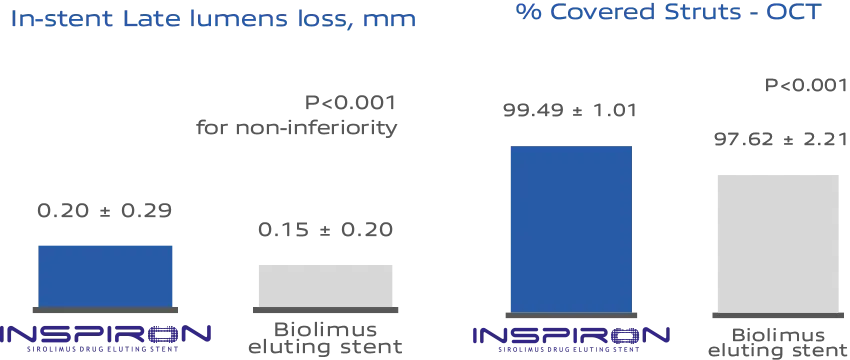
The Inspiron stent demonstrates in-stent late luminal loss that is non-inferior to that of Biolimus-eluting stents’, while exhibiting superior strut coverage in OCT (Optical Coherence Tomography) analysis compared to
Biolimus-eluting stents (p<.001) at 9-months²
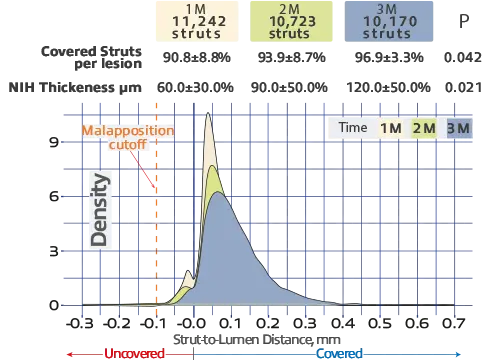
The Inspiron stent exhibited an impressive strut coverage pattern of over 90% within the first month, achieving 97.21% coverage by the third month³.
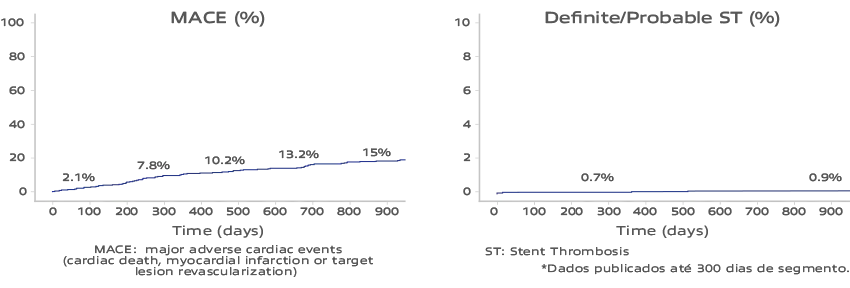
The Inspiron stent has demonstrated proven safety and efficacy, characterized by low rates of thrombosis in long-term follow-up studies’-s
Inspiron Real Life I – Novel DES in high-risk patients3
900 days of median follow-up [n=470]*
Destiny Trial:
Safety proven over 5 years
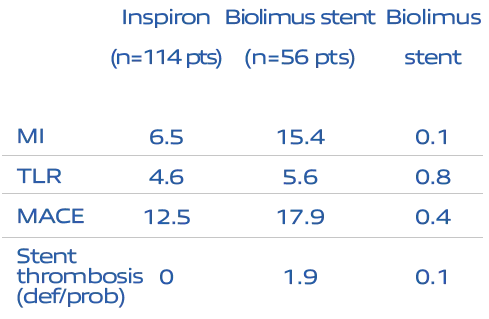
Table of Size and Catalogue Number
Length
| Diameter | 9mm | 13mm | 16mm | 19mm | 23mm | 29mm | 33mm | 38mm | 48mm | 58mm |
| 2.25mm | 128032 | 128034 | 128035 | 128036 | 128037 | |||||
| 2.5mm | 128040 | 128041 | 128042 | 128043 | 128044 | 128045 | 128046 | 128047 | ||
| 2.75mm | 128050 | 128051 | 128052 | 128053 | 128054 | 128055 | 128056 | 128057 | ||
| 3.0mm | 128060 | 128061 | 128062 | 128063 | 128064 | 128065 | 128066 | 128067 | 128068 | 128069 |
| 3.5mm | 128070 | 128071 | 128072 | 128073 | 128074 | 128075 | 128076 | 128077 | 128078 | 128079 |
| 4.0mm | 128080 | 128081 | 128082 | 128083 | 128084 | 128085 |
Not available in Europe, North America and Asia
R01 – 2025
References
¹Lemos PA, et al. Cardiovasc Ther. 2015 Dec;33(6):367-71. DESTINY Randomized Trial; ²Costa JR Jr, et al. Int J Cardiovasc Imaging. 2017 Feb;33(2):161-
-168. IVUS and OCT results of the DESTINY trial.; ³Silva GBG, et al. J Invasive Cardiol. 2023 May;35(5):E225-E233. Epub 2023 Mar 13. REPAIR Trial.; ⁴Prado
GFA Jr, et al. Rev Port Cardiol (Engl Ed). 2021 Feb;40(2):71-76. Five-year results of the DESTINY randomized trial.; 5Inspiron Real Life I – Novel DES in
high-risk patients (n=470; 900 dias médios de seguimento) – Dados publicados somente até 300 dias de segmento.
R01 – 2025